LILBID Time-of-Flight Mass Spectrometer
Fig. 1 shows the principle of a LILBID experiment. You either have a liquid beam of small diameter or a train of droplets flying through vacuum. The liquid beam or the flying droplets are intercepted by a pulsed infrared laser operating at the absorption wavelength of the liquid.
When the liquid explodes, biological molecules contained within the liquid are set free usually with a small number of charges and some solvent molecules on it. They are detected in a time-of-flight mass spectrometer.
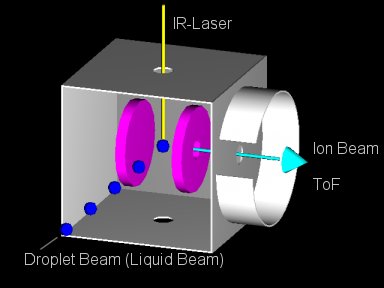
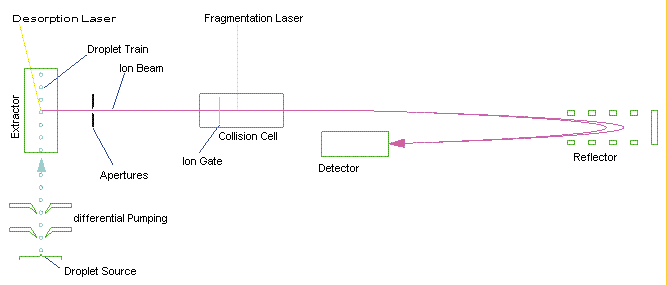
Fig. 2 shows the schematics of such a mass spectrometer. As in fig. 1, a liquid beam or a train of droplets is intercepted by a laser that vaporates the solvent and liberates biological molecules and noncovalently bound complexes. These are accelerated into the mass spectrometer. The fragmentation laser can be used e.g. to probe the nature of bonds in noncovalently bound complexes. The gridless reflector and detector are used to detect the ions with high senitivity and high mass resolution.
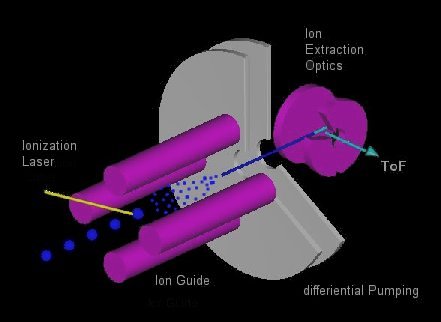
Fig. 3 shows a possible extension of such a LILBID mass spectrometer. Liquid droplets are shot into an ion guide, where they are vaporized by an infrared laser. The ions created are transported by the ion guide into the extraction optics of the mass spectrometer. Producing the ions within the ion guide allows all the standard interactions done within ion guides such as cooling and heating.